Background: BCR::ABL1 tyrosine kinase inhibitors (TKIs) in combination with chemotherapy or steroids remain the standard of care in patients with newly diagnosed Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). Most patients treated with first- or second-generation TKIs eventually experience disease progression due to treatment resistance. Ponatinib is the only currently approved pan-BCR::ABL1 inhibitory TKI that potently inhibits wild-type and single resistance mutation variants of BCR::ABL1, including T315I. Multiple studies have shown promising minimal residual disease (MRD) negativity (neg) rates and survival outcomes with ponatinib in combination with chemotherapy or chemotherapy-free regimens. PhALLCON (NCT03589326) is the first randomized study comparing frontline TKIs in adults with Ph+ ALL. The primary endpoint for PhALLCON was met, with a clinically significantly higher MRD-neg complete remission (CR) rate at end of induction (EOI) with ponatinib vs imatinib (34.4% vs 16.7%; risk difference: 0.18 [95% confidence interval (CI): 0.06‒0.29]; P=0.0021) and a manageable safety profile comparable with imatinib. Here we report subgroup efficacy analyses from PhALLCON.
Methods: This global, registrational, phase 3, open-label study randomized newly diagnosed adult patients with Ph+ ALL 2:1 to receive ponatinib (30 mg once daily [QD]) or imatinib (600 mg QD) plus reduced-intensity chemotherapy through EOI (Cycles 1-3), consolidation (Cycles 4-9), and post-consolidation (Cycles 10-20). Following post-consolidation, patients continued to receive single-agent ponatinib or imatinib until disease progression or unacceptable toxicity. The composite primary endpoint was MRD-neg ( BCR:: ABL1 ≤0.01% [MR4])CR for 4 weeks at EOI. Event-free survival (EFS; any-cause death, failure to achieve CR by EOI, relapse from CR) was a key secondary endpoint. A post-hoc analysis of progression-free survival (PFS; EFS-defined events, failure to achieve MRD-neg by the end of treatment, and loss of MRD-neg) was conducted. Subgroup analyses were performed relative to baseline demographic and disease characteristics.
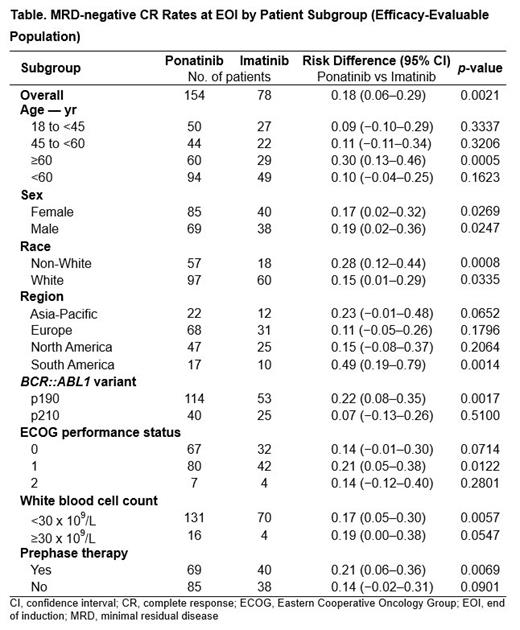
Results: Of 245 randomized patients, 232 (ponatinib, n=154; imatinib, n=78) had baseline BCR::ABL1 p190/p210 variants verified by central lab (efficacy-evaluable population); median age was 54 years (37.1% ≥60 years). As of Aug 2022, 78 patients (ponatinib/imatinib: 68 [41.5%]/10 [12.3%]) remained on study treatment; the top 3 reasons for discontinuation were hematopoietic stem cell transplantation (HSCT; 30.5%/37.0%), adverse events (12.2%/12.3%), and lack of efficacy (7.3%/25.9%). Median follow-up in the ponatinib and imatinib arms was 20 months and 18 months, respectively. Benefit for ponatinib was observed across all subgroups analyzed ( Table). All age subgroups had higher rates of MRD-neg CR with ponatinib vs imatinib, with the greatest benefit observed in patients ≥60 years (40.0% ponatinib vs 10.3% imatinib; P=0.0005). MRD-neg CR rate was higher for ponatinib vs imatinib among those with the BCR::ABL1 p190 variant (38.6%/17.0%; P=0.0017); it was higher for the p210 variant as well, but not significantly (22.5% vs 16.0%; P=0.51). Median PFS was longer with ponatinib vs imatinib regardless of age, with the greatest difference observed in the subgroup of patients ≥60 years (22.5 months for ponatinib vs 8.3 months for imatinib; hazard ratio: 0.594 [95% CI: 0.332-1.063]).
Conclusions: Ponatinib was superior to imatinib in combination with reduced-intensity chemotherapy in the front-line setting for patients with Ph+ ALL, with a clinically significantly higher MRD-neg CR rate at EOI. Benefit was observed across all subgroups, particularly for patients ≥60 years and for those with the BCR::ABL1 p190 variant.
Disclosures
Aldoss:Jazz: Consultancy; Sobi: Consultancy; Pfizer: Consultancy; KiTE: Consultancy; Amgen: Consultancy, Honoraria; Takeda: Consultancy. Ribera:AMGEN: Research Funding; Takeda: Consultancy; Novartis: Consultancy; Bristol Myers Squibb: Consultancy; Pfizer: Consultancy, Research Funding; Incyte: Consultancy, Research Funding. Kantarjian:Bristol-Myers Squibb (Inst): Research Funding; Novartis (Inst): Research Funding; Daiichih-Sankyo (Inst): Honoraria, Research Funding; AstraZeneca/MedImmune: Honoraria; Ipsen: Honoraria; Precision Biosciences: Honoraria; Novartis: Honoraria; Immunogen (Inst): Honoraria, Research Funding; Astellas Pharma: Honoraria; Ascentage Pharma Group: Honoraria; Jazz Pharmaceuticals (Inst): Honoraria, Research Funding; Taiho Pharmaceutical: Honoraria; Shenzhen Target Rx: Honoraria; KAHR Medical: Honoraria; Abbvie (Inst): Research Funding; Amgen (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding; Pfizer: Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Honoraria. Montesinos:Astellas: Consultancy, Speakers Bureau; GILEAD: Consultancy; BMS: Consultancy, Other, Research Funding; Celgene: Consultancy; Janssen: Speakers Bureau; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding, Speakers Bureau; Ryvu: Consultancy; Jazz pharma: Consultancy, Research Funding, Speakers Bureau; Menarini-Stemline: Consultancy, Research Funding; BEIGENE: Consultancy; INCYTE: Consultancy; Abbvie: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy, Research Funding; Kura oncology: Consultancy; OTSUKA: Consultancy; NERVIANO: Consultancy; Daiichi Sankyo: Consultancy, Research Funding. Leonard:Pfizer: Consultancy; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Kite/Gilead: Consultancy; Takeda: Consultancy. Gomez-Almaguer:AMGEN: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Novartis: Honoraria; AbbVie: Consultancy, Honoraria. Baer:Kite, a Gilead company (Inst): Research Funding; FORMA Therapeutics (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding; Abbvie (Inst): Research Funding; Kura Oncology (Inst): Research Funding; Takeda (Inst): Research Funding. McCloskey:Bristol-Myers Squibb/Pfizer: Consultancy, Honoraria, Speakers Bureau; Amgen: Speakers Bureau; BluPrint Oncology: Honoraria; Blueprint Medicines: Consultancy; Incyte: Speakers Bureau; Jazz Pharmaceuticals: Speakers Bureau; Novartis: Consultancy; BluePrint Health: Speakers Bureau; Stemline Therapeutics: Speakers Bureau; Takeda: Speakers Bureau. Minami:Taiho Pharmaceutical: Research Funding; Takeda: Research Funding; Tejin Pharma: Research Funding; Sumitomo Pharma Oncology: Research Funding; Shionogi: Research Funding; Sanofi: Research Funding; Otsuka: Research Funding; Nippon Shinyaku: Research Funding; Nihonkayaku: Research Funding; Kyowa Hakko Kirin: Research Funding; Dainippon Sumitomo Pharma: Research Funding; Asahi Kasei: Research Funding; Taiho Pharmaceutical: Honoraria; Shinogi: Honoraria; Sanofi: Honoraria; Otsuka: Honoraria; Ono Pharmaceutical: Honoraria; Merck: Honoraria; Meiji Seika Kaisha: Honoraria; Lilly: Honoraria, Research Funding; Kyowa Hakko Kirin: Honoraria; Eisai: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Chugai Pharma: Honoraria, Research Funding; Bayer: Honoraria, Research Funding; Abbvie: Honoraria; Pfizer Japan Inc.: Honoraria; Bristol-Myers Squibb Company: Honoraria; Takeda: Honoraria; Novartis Pharma KK: Honoraria. Rousselot:Takeda: Consultancy; Novartis: Consultancy; Bristol Myers Squibb: Consultancy; Pfizer: Consultancy; Incyte: Consultancy. Vachhani:Cogent Biosciences: Consultancy; Incyte: Consultancy, Speakers Bureau; CTI BioPharma Corp: Consultancy, Speakers Bureau; Daiichi Sankyo: Consultancy; GlaxoSmith Kline: Consultancy; Karyopharm: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Genentech: Consultancy; Servier: Consultancy; Stemline: Consultancy; MorphoSys: Consultancy; LAVA therapeutics: Consultancy; Blueprint Medicines: Consultancy, Speakers Bureau; Amgen: Consultancy; AbbVie: Consultancy. Wang:Takeda: Consultancy; Dava oncology: Speakers Bureau; PharmaEssentia: Consultancy; Kura Oncology: Speakers Bureau; Abbvie: Consultancy; Astellas: Consultancy, Speakers Bureau; Kite: Consultancy, Speakers Bureau; GlaxoSmithKline: Consultancy; Pfizer: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; BMS: Consultancy; Gilead: Consultancy; Jazz: Consultancy. Hennessy:Takeda: Current Employment. Patel:Takeda: Current Employment. Vorog:Takeda: Current Employment. Wang:Takeda: Current Employment. Jabbour:Genentech: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding; Adaptive Biotech: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal